PODS促进治疗性蛋白传递:PODSBMP-2刺激体内骨再生
PODS® facilitates therapeutic protein delivery: PODS® BMP-2 stimulates bone regeneration in vivo
Data Courtesy of Hajime Mori, Kyoto Institute of Technology, Kyoto, Japan
Introduction to Sustained Release Growth Factors: PODS®
The challenge with soluble growth factors
Many proteins, especially growth factors and cytokines, when used as a reagent, degrade quickly, rapidly losing their bioactivity. This fragility hampers research and significantly limits the therapeutic potential of proteins.
Protein Micro-depots
Development of a technology that can continuously replenish active protein from a local, microscopic store has been a significant challenge, but one that could transform the fields of cell culture and medicine by allowing greater control over the growth of cells.
Introducing PODS®
PODS® technology has made the goal of a micro-depot for proteins a reality. PODS® is a sustained release system which continuously replenishes proteins from millions of local microscopic stores which can be placed next to (or at a distance from) cells, either randomly or in precise locations. Just like cells, these micro-depots release a steady stream of bioactive protein. This protein can be limited to local surroundings or dispersed more widely, or made to form a gradient.
How does it work?
At the heart of PODS® is an extraordinary polyhedrin protein. This specific polyhedrin protein has the unique ability to encase cargo proteins within perfect, transparent, cubic, micro-sized crystals, much smaller than the cells. These protein crystals form admixtures of the polyhedrin and cargo proteins which slowly degrade releasing the biologically active cargo protein.
How can PODS® help my research?
PODS® are tough and will withstand physical and chemical stress, so you can handle them with ease. PODS® can be made to release intact cargo protein over days, weeks or even months. Using PODS® you can readily create a steady-state protein environment in microscopic detail wherever you want, tailored exactly to your requirements. This is the power of PODS®. PODS® proteins are now available for many growth factors and cytokines and are already being used in many leading world-class research labs. PODS® protein applications include:
- Micropatterning
- Physiological, stable gradient formation
- Bioinks for 3D printing
- Microcarriers
- Functionalizing scaffolds
- Microfluidics (lab on a chip)
- Improved and simplified stem cell culture
- Therapeutic protein delivery
Methods
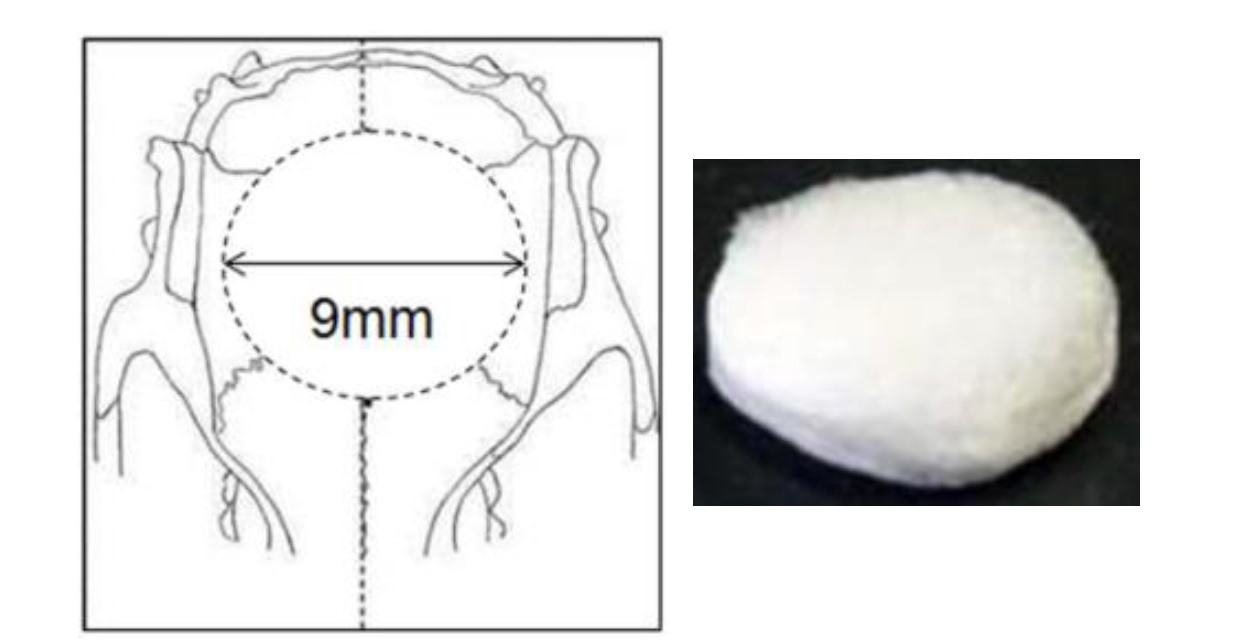
Bone defect: A 9 mm disc of rat calvaria bone was removed with a trephine bur. Two doses each (high and low) of either PODS® BMP-2 crystals, PODS® Empty crystals or standard rhBMP-2 were soaked into absorbable atelocollagen sponges (ACS) and implanted into the bone defects. The regeneration of bone was monitored with x-ray radiography in intervals for up to 15 weeks. NOTE: a single application of PODS® crystals was used.
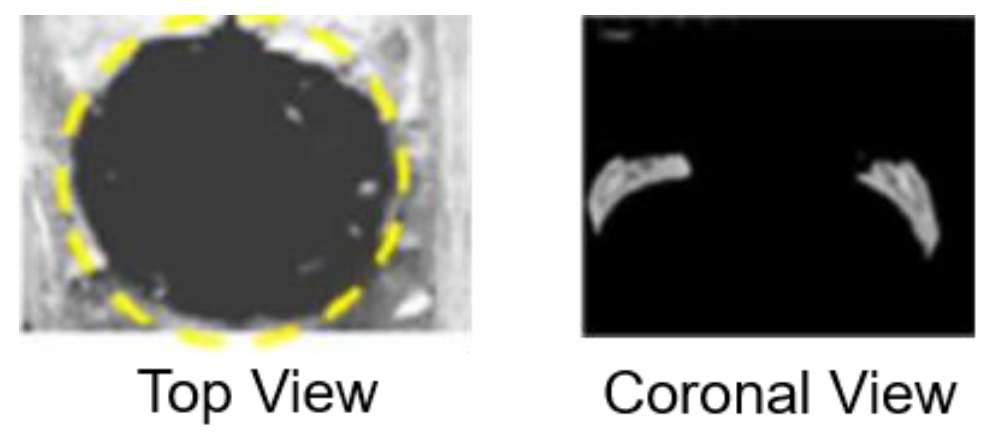
Bone volume measurement: μ-CT analysis was performed to visualize and quantify bone regeneration. The 3-D images were reconstructed to illustrate the top and coronal section views. Newly generated bone was evaluated by bone volume in bone defects assessed from the projected area of the μ-CT images.
Results
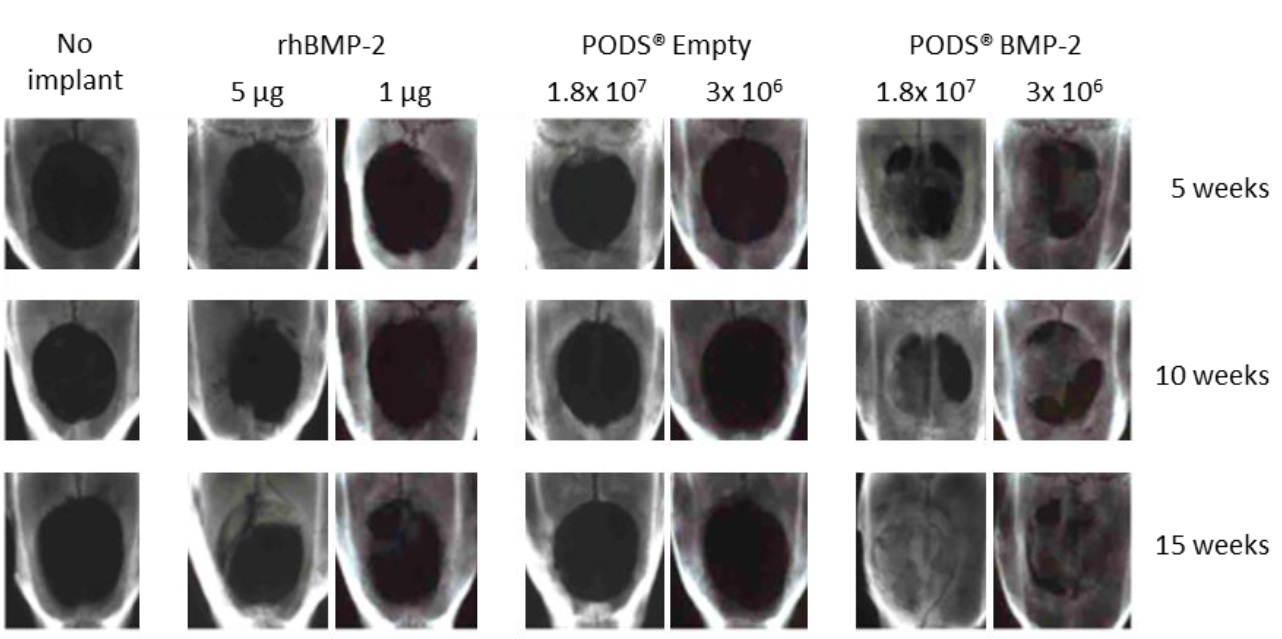
X-ray micrographs of calvarial bone defect monitored for up to 15 weeks. After implantation of treated ACS sponges, bone regeneration was followed for a period of up to 15 weeks. PODS® BMP-2 at low and high doses produced significantly more bone reformation at all time points than standard rhBMP-2, PODS® Empty or untreated bone defect. Upon resection, crystals were still present up to 10 weeks but were completely degraded at 15 weeks after implantation. No inflammatory response was observed (adapted from Matsumoto, G. et al. Sci. Rep. 2, 935; DOI:10.1038/srep00935 (2012), licensed under CC by-nc-sa 3.0).
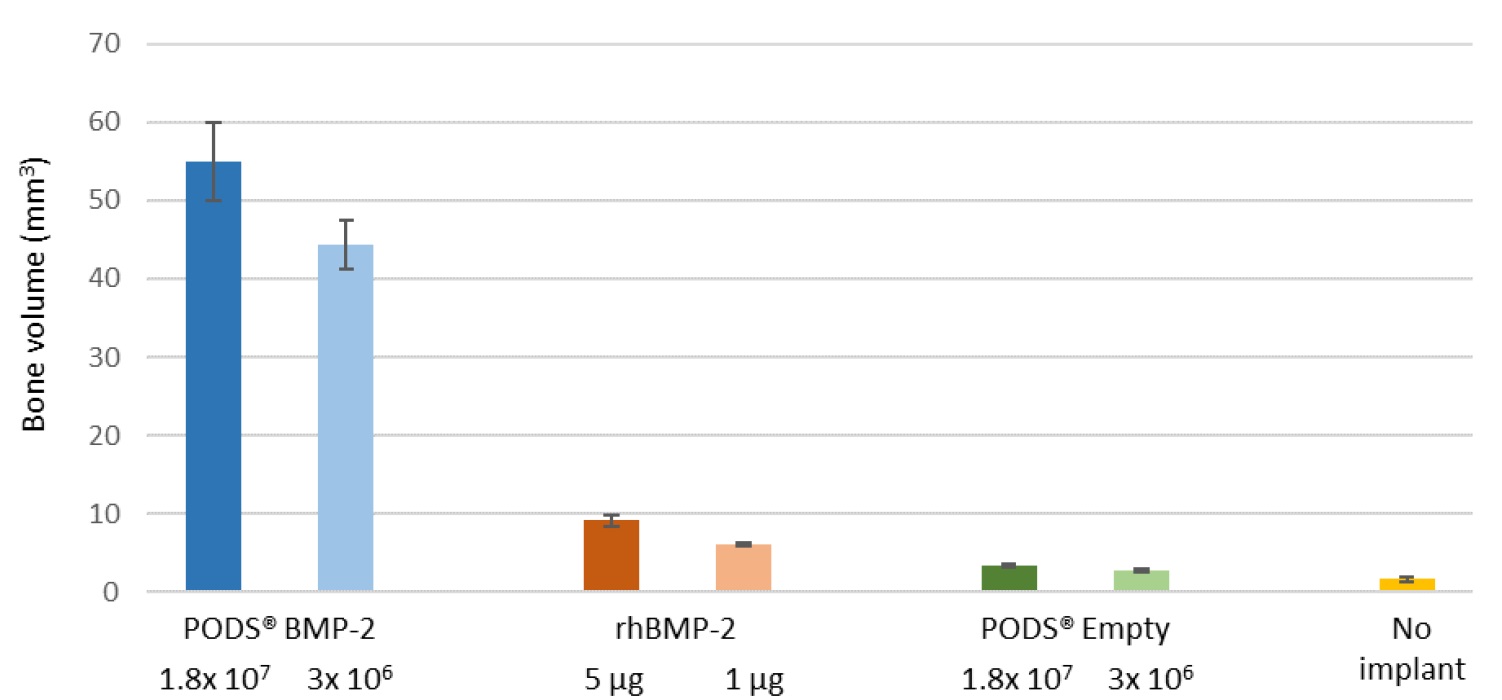
Bone volume measurement in critical-sized calvarial defect 15 weeks after implantation. Levels of bone regeneration was measured by analyzing μ-CT images. Corroborating the visual inspection of the x-ray micrographs (Figure A), both doses of PODS® BMP-2 treatment stimulated significantly more bone growth over 15 weeks than either standard rhBMP-2, PODS® Empty crystals or no intervention (recreated from Matsumoto, G. et al. Sci. Rep. 2, 935; DOI:10.1038/srep00935 (2012), licensed under CC by-nc-sa 3.0
Conclusions
- PODS® crystals can deliver growth factors localized in vivo and is therefore ideal for therapeutic use.
- A single application of PODS® crystals is effective in vivo and delivers therapeutic proteins for several weeks.
- PODS® growth factors are more efficacious at lower doses compared to standard recombinant growth factor treatments further reducing off-site side effects.

 0
0



